

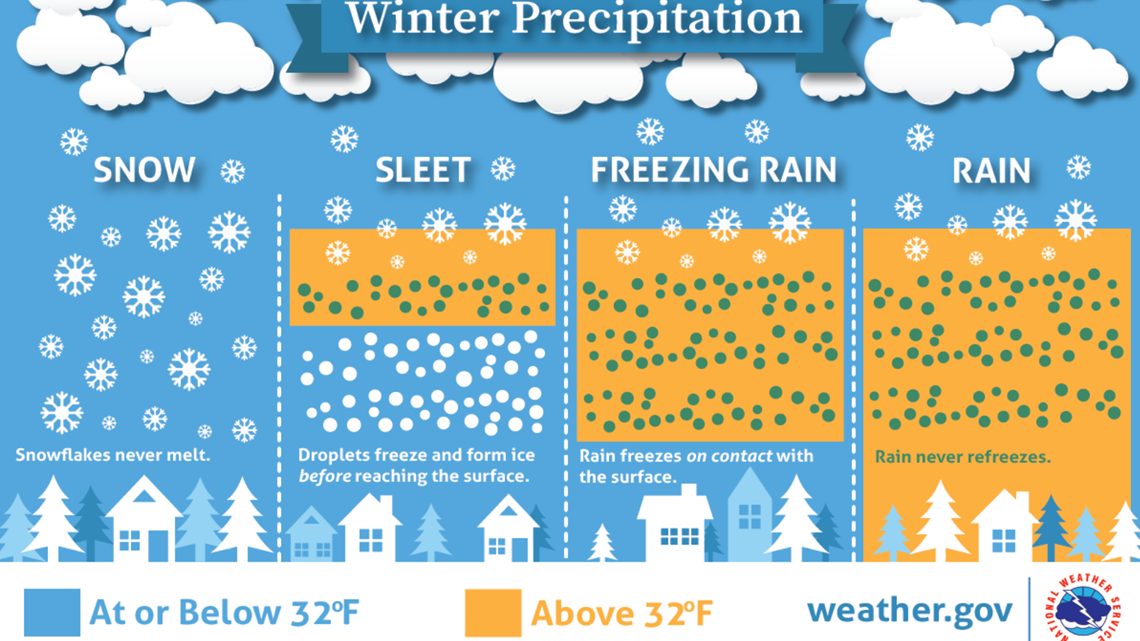
Once we know that = 3.0 x 10-6 we can find the pOH (-log (3.0 x10 -6) to be 5.52 Where the equilibrium expression is Kb = / = 3.0 x 10 -6īut since the =, you can cancel them out of the Kb expression above, leaving: So, what you have is a mixture of a base and it's conjugate acid and they will form the following equilibrium: In case of an inorganic chemical reaction leading to precipitation, the chemical reagent causing the solid to form. Since the moles are dissolve in a total volume of 125 ml, the =. The solid formed is called the precipitate. Examine this figure and consider all the processes that have to occur to cause rain, snow, or hail. 005 mol and the moles of the conjugate acid N2H5+ will also be. Precipitation is the process whereby liquid droplets of water (raindrops), solid bits of ice (snowflakes and hail), or some combination of these fall from the sky. This means that you have half neutralized the base so the moles of N2H4 remaining will be. 005 mol of HNO3 and each mol of HNO3 neutralized 1 mol of N2H4 and formed 1mole of N2H5+ (. The balanced equation for the titration is: Calculate the pH of the resulting solution after 25.0 mL of HNO3 is added. Well, let's give the second problem a shot:Ģ. I'll tackle problem in a separate post in a bit. Therefore, the concentration of the ions is NOT large enough to cause a precipitate of Mg(OH)2 to form. We see that Ksp > Qsp and, hence, no precipitate forms (the reaction goes to the right-everything. So in this case the products of the ions is less than the Ksp value: Here, we calculate Qsp (0.0024)(1.0 x 10-6) 2.4 x 10-9. Multiplying the ions as in the Ksp equation produces So once they are mixed, the = 2.0 x 10 -4 while the = 1.0 x 10 -4.
When the solutions are mixed the concentrations will be cut in half due to the fact that the volume of the solution has been doubled. Will a precipitate form when 100.0 mL of 4.0 x 10^-4 M mg(NO3)2 is added to 100.0 mL of 2.0 x 10^-4 M NaOH. If the product of the concentration of the ions is less than the Ksp value, no precipitate will form. Now if the product of the concentration of the ions raised to the power of their coefficients exceeds the Ksp value then the system is not at equilibrium and ions will precipitate out. (I looked up the value for the Ksp for Mg(OH)2 and found it here: The solubility product (Ksp )expresses the relationship between the ions at equilibrium (when you have a saturated solution).

The reaction for the dissolving of Mg(OH)2 is However, even slightly soluble substances like Mg(OH)2 dissolve a bit and go to equilibrium with their ions. Well Sunil, you are correct that the Mg(OH)2 is the precipitate.


 0 kommentar(er)
0 kommentar(er)
